The rich get richer: synaptic remodeling between climbing fibers and Purkinje cells in the developing cerebellum begins with positive feedback addition of synapses
BioRxiv, 2019.
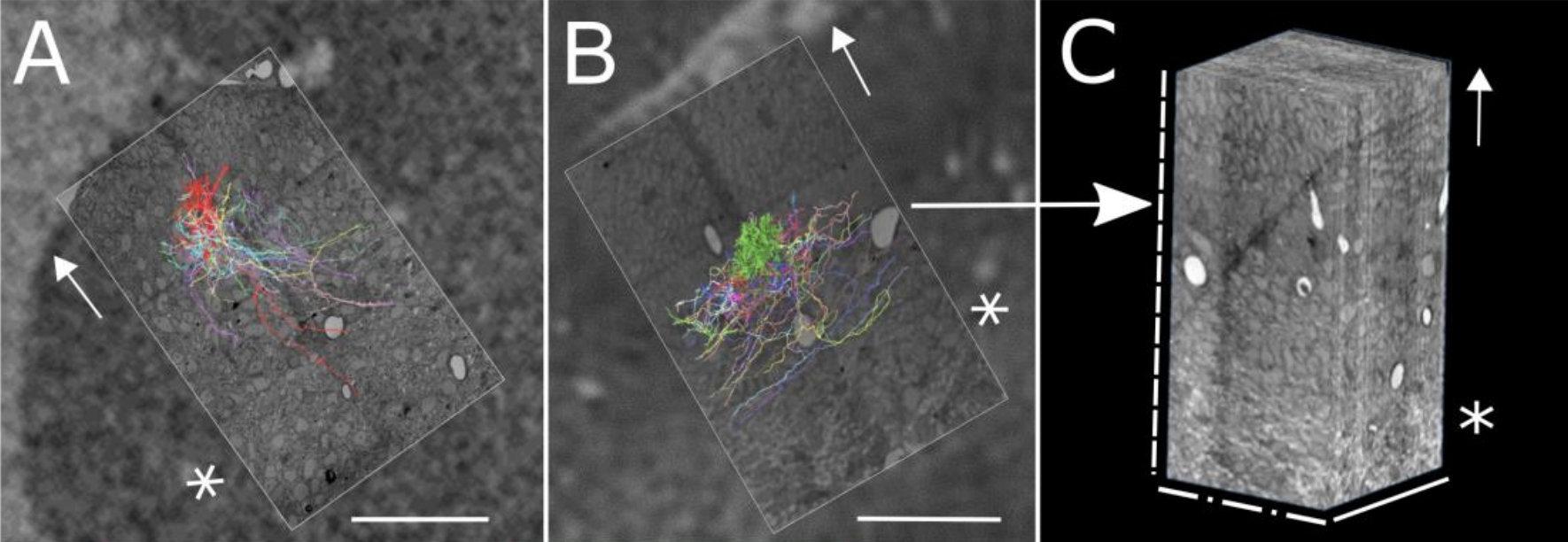
During postnatal development, cerebellar climbing fibers strongly innervate a subset of their original Purkinje cell targets and eliminate their connections from the rest. In the adult, each climbing fiber innervates a small number of Purkinje cells and each Purkinje cell is innervated by a single climbing fiber. To get insight about the processes responsible for this remapping, we reconstructed serial electron microscopy datasets from mice during the first postnatal week. In contrast to adult connectivity, individual neonatal climbing fibers innervate many nearby Purkinje cells, and multiple climbing fibers innervate each Purkinje cell. Between postnatal days 3 and 7, Purkinje cells retract long dendrites and grow many proximal dendritic processes. On this changing landscape, individual climbing fibers selectively add many synapses to a subset of Purkinje cell targets in a positive-feedback manner, without pruning synapses from other Purkinje cells. The active zone sizes of synapses associated with powerful versus weak inputs are indistinguishable. These results show that changes in synapse number rather than synapse size are the predominant form of early developmental plasticity. Finally, although multiple climbing fibers innervate each Purkinje cell in early postnatal development, the number of climbing fibers and Purkinje cells in a local cerebellar region nearly match. Thus, initial over-innervation of Purkinje cells by climbing fibers is economical, in that the number of axons entering a region is enough to assure that each axon ends up with a postsynaptic target, and that none branched there in vain.