Lymphocyte networks are dynamic cellular communities in the immunoregulatory landscape of lung adenocarcinoma
Cancer Cell, 2023.
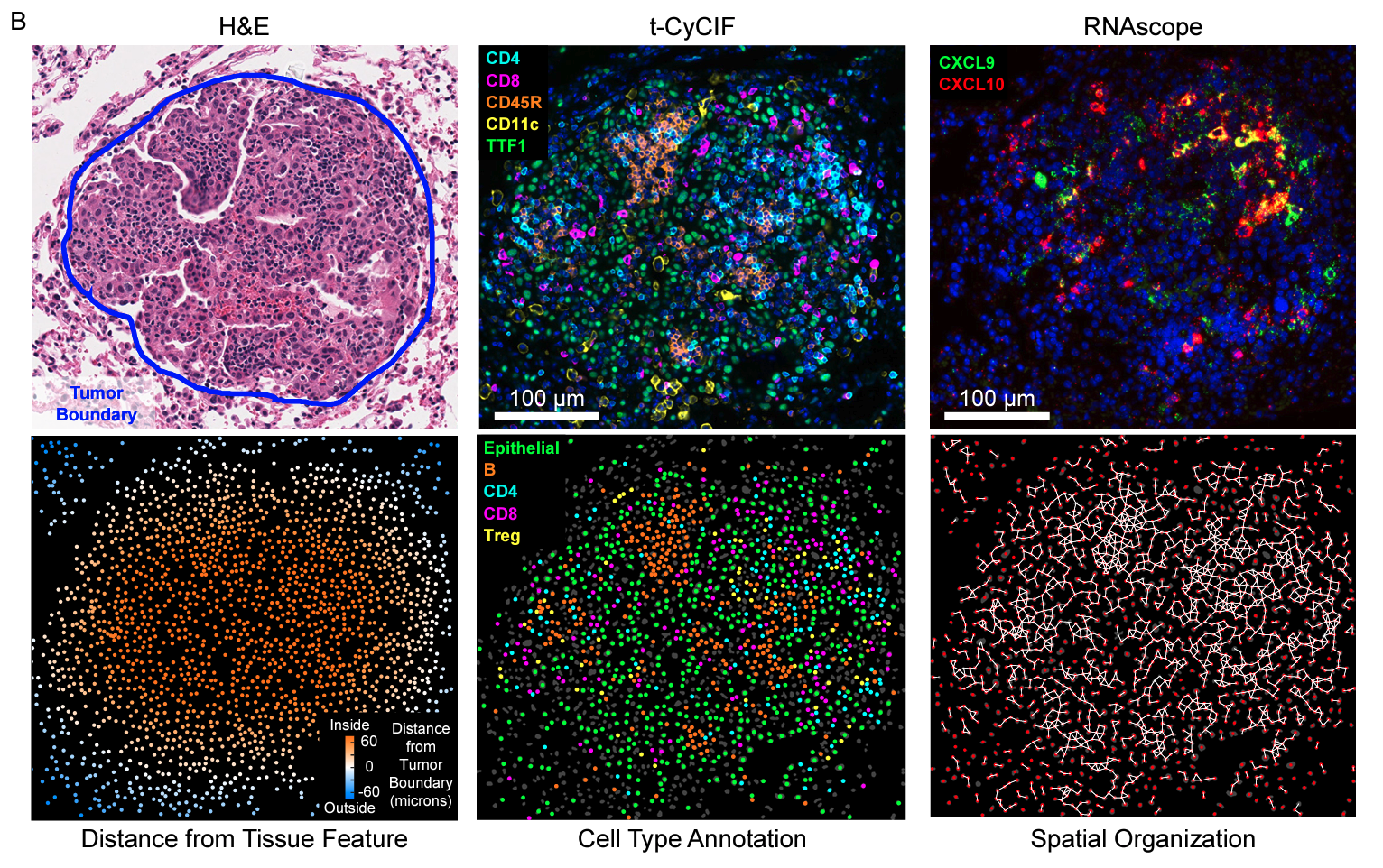
Summary Lymphocytes are key for immune surveillance of tumors, but our understanding of the spatial organization and physical interactions that facilitate lymphocyte anti-cancer functions is limited. We used multiplexed imaging, quantitative spatial analysis, and machine learning to create high-definition maps of lung tumors from a Kras/Trp53-mutant mouse model and human resections. Networks of interacting lymphocytes (“lymphonets”) emerged as a distinctive feature of the anti-cancer immune response. Lymphonets nucleated from small T cell clusters and incorporated B cells with increasing size. CXCR3-mediated trafficking modulated lymphonet size and number, but T cell antigen expression directed intratumoral localization. Lymphonets preferentially harbored TCF1+ PD-1+ progenitor CD8+ T cells involved in responses to immune checkpoint blockade (ICB) therapy. Upon treatment of mice with ICB or an antigen-targeted vaccine, lymphonets retained progenitor and gained cytotoxic CD8+ T cell populations, likely via progenitor differentiation. These data show that lymphonets create a spatial environment supportive of CD8+ T cell anti-tumor responses.
Acknowledgements
This work was supported by NIH grants R01-CA194005 (S.S.), R41-CA224503 (P.K.S.), U54CA225088 (P.K.S., S.S.), T32-GM007748 (S.C.), and T32-HL007627 (G.G.), by the Bridge Project, a partnership between the Koch Institute for Integrative Cancer Research at MIT and the Dana-Farber/Harvard Cancer Center (P.K.S., S.S., T.J.), the Howard Hughes Medical Institute (T.J.), the Ludwig Center at Harvard (P.K.S., S.S.), the American-Italian Cancer Foundation postdoctoral fellowship (G.G.), K99-CA256497 (A.J.N), fellowship awards from the Jane Coffin Childs Memorial Fund for Medical Research and the Ludwig Center for Molecular Oncology at MIT (M.L.B.), and the BWH President’s Scholar Award (S.S.). We thank Dana-Farber/Harvard Cancer Center for the use of the Specialized Histopathology Core, which provided histopathology services. Dana-Farber/Harvard Cancer Center is supported in part by an NCI Cancer Center Support Grant P30-CA06516. This work was supported in part by the Koch Institute Support (core) Grant P30-CA014051 from the National Cancer Institute. T.J. is a Daniel K. Ludwig Scholar.